
Riverside, CA 92507
(P) (951) 823-8490
(F) (951) 823-8495
(E) info@cibaria-intl.com
Specification Sheet
Label Ingredients Statement:Apricot Kernel Oil, Expeller Pressed, Refined
Product Description: Apricot Kernel Oil is produced from the apricot kernels (Prunus Armeniaca). These kernels contain about 40-50% fatty oil which is gained by careful mechanical pressing of selected kernels and ensuing filtration. The viscous clear oil is of a light-yellow to ochre color with a typical mild odor and taste.
- Similar to almond oil and peach oil
- Composed of oleic acid and linoleic acid
- Can be used on the scalp to improved its condition
- Contains vitamin E
- Used in cosmetics to soften skin
- Used to manufacture soaps and cold creams
Prop 65 Statement:
Allergen Disclosure
Statement: Apricot Kernel Oil, Expeller Pressed, Refined does not contain any of the following allergens, sensitive ingredients, or restricted ingredients:
Egg/egg products
Shellfish/shellfish products
Other legumes
Gluten
Animal fat/oil
Hydrolyzed vegetable protein
Sulfites
BMA
FD&C colors
Food Starch
Fish/fish products
Mollusks
Soy/soybeans/soy products
Wheat/wheat products
Lecithin
Hydrolyzed animal protein
Yeast Extract
Monosodium Glutamate
BHT
Natural Colors
Maltodextrin
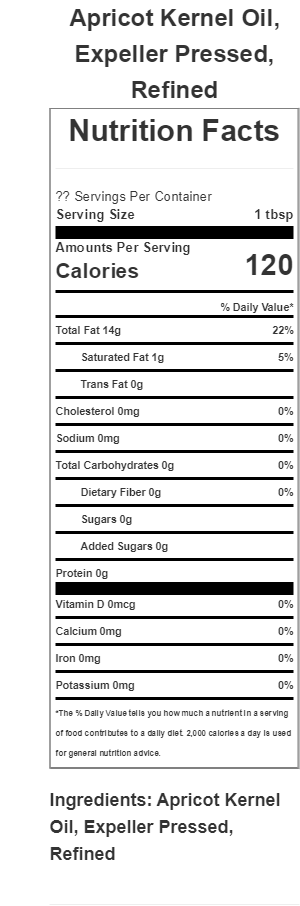
6% Saturated Fat
60% Monounsaturated Fat
29% Polyunsaturated Fat
Storage: Light protected, not above room temperature, in tightly sealed containers (nitrogen blanketed is best).
Shelf Life: Shelf life is 15 months from date of production. Cibaria guarantees a minimum of 6 months from date of shipment.
Sewer Sludge and Irradiation Statement: Cibaria International Products are food grade and have not had any contact with sewage sludge or radiation.
Applications For Product: In cosmetics as a base oil for skin oils and creams where its stability is much appreciated (low rancidity). Likewise, Apricot Kernel Oil is used in pharmaceuticals and as a choice cooking oil.
Country of Origin: Italy, USA.
USDA NDB (National Nutrition Database)
| Nutrient | Unit | Value per 100.0g | Tbsp 13.5g |
| Proximates | |||
| Water | g | 0 | 0 |
| Energy | kcal | 884 | 119 |
| Protein | g | 0 | 0 |
| Total lipid (fat) | g | 100 | 13.5 |
| Carbohydrate, by difference | g | 0 | 0 |
| Fiber, total dietary | g | 0 | 0 |
| Sugars, total | g | 0 | 0 |
| Minerals | |||
| Calcium, Ca | mg | 0 | 0 |
| Iron, Fe | mg | 0 | 0 |
| Magnesium, Mg | mg | 0 | 0 |
| Phosphorus, P | mg | 0 | 0 |
| Potassium, K | mg | 0 | 0 |
| Sodium, Na | mg | 0 | 0 |
| Zinc, Zn | mg | 0 | 0 |
| Vitamins | |||
| Vitamin C, total ascorbic acid | mg | 0 | 0 |
| Thiamin | mg | 0 | 0 |
| Riboflavin | mg | 0 | 0 |
| Niacin | mg | 0 | 0 |
| Vitamin B-6 | mg | 0 | 0 |
| Folate, DFE | µg | 0 | 0 |
| Vitamin B-12 | µg | 0 | 0 |
| Vitamin A, RAE | µg | 0 | 0 |
| Vitamin A, IU | IU | 0 | 0 |
| Vitamin E (alpha-tocopherol) | mg | 4 | 0.54 |
| Vitamin D (D2+D3) | µg | N/A | N/A |
| Vitamin D | IU | N/A | N/A |
| Vitamin K (phylloquinone) | µg | N/A | N/A |
| Lipids | |||
| Fatty acids, total saturated | g | 6.3 | 0.851 |
| Fatty acids, total monounsaturated | g | 60 | 8.103 |
| Fatty acids, polyunsaturated | g | 29.3 | 3.957 |
| Cholesterol | mg | 0 | 0 |
| Other | |||
| Caffeine | mg | 0 | 0 |
Organoleptic Characteristics: |
|
| Appearance/Clarity | Viscous clear, light yellow |
| Flavor/Odor | Typical |
| Color (Lovibond) Red | N/A |
| Color (Lovibond) Yellow | N/A |
Typical Analysis Ranges: |
|
| Free Fatty Acid (% m/m expressed in oleic acid) | N/A |
| Moisture | N/A |
| Peroxide Value | N/A |
| Iodine Value | 90-115 |
| Saponification Value | 185-195 |
| p-Anisidine Value | N/A |
| Cold Test | N/A |
| Refractive Index | (20°C) 1.469-1.475 |
| Specific Gravity | N/A |
| Oil Stability Index(OSI) | N/A |
| Smoke Point | N/A |
| Additives | N/A |
Typical Fatty Acid Ranges: |
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
Registrations and Other Product Information:
CAS 72869-69-3
EINCS 272-046-1
INCL: Prunus Armeniaca Kernel Oil
Notes: Kosher. Density (20°C) - 0.91-0.923. Acid Value, Refined - Max 0.6. This product is not affected by the GMO problem, therefore it does not need to be labeled regarding any genetic modification as per the new GMO regulations (1829/2003 and 1830/2003).

This specification was developed with the utmost care based on up-to-date information available, but should be scrutinized by the recipient. It does not release him or her from checking the quality of goods delivered with proper diligence.
Reviewed: 1/25/19
Revised: 1/25/19